What is ICH E6?
It is the International Council for Harmonization’s (ICH) guideline for Good Clinical Practice (GCP). The guideline establishes an international standard for the design, conduct, recording, and reporting of clinical trials involving human participants.
The Guidance is centered on its Principles, which provide a high-level, flexible framework for conducting a clinical trial and are supplemented by the annexes. They are intended to provide guidance for all stages of clinical trials in human participants.
ICH E6 R3: What’s New?
The release of ICH E6 R3, the most recent update to the Guideline for Good Clinical Practice, has introduced some significant changes from what was seen in the prior versions. The key changes are centered around the reorganization of the principles as a whole with the total number of principles changing from 13 to 11. This change was achieved through the consolidation of five of the key principles from R2 into the first principle of R3, and two new principles, 7 and 10, being introduced. The key content changes from prior versions of E6 include: (1) clarifying the role risk should play in study design; (2) more clearly defining roles and responsibilities in a study; (3) incorporating quality principles in study design; (4) making protocols more clear and concise; and (5) improved data generation, study transparency and IP Management. More details on these changes has been outlined below.
The Two New Principles:
-
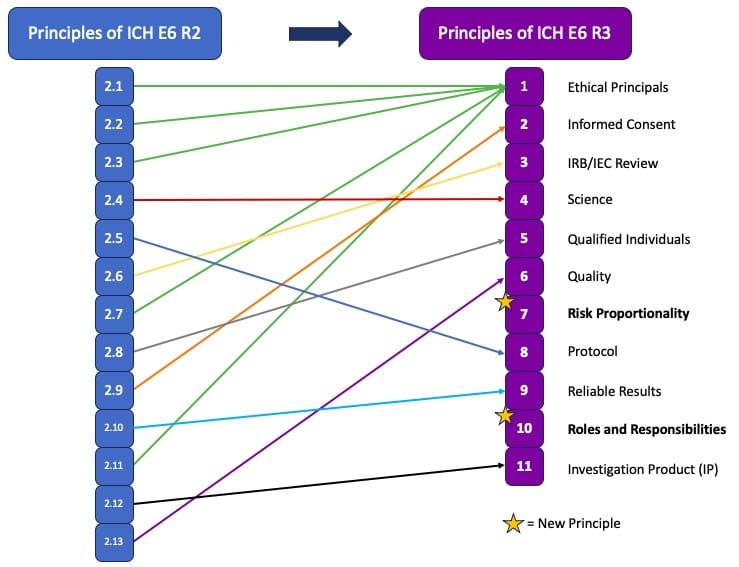
Principle 7: Describes Risk Proportionality, aligns with the guidelines of E8, the General Considerations for Clinical Studies, and advocates for the implementation of clinical trial elements that are proportionate to both the risks to participants and to the importance of the data collected. Additionally clinical studies should also be proactive and consistent in their risk management. This principle speaks to avoiding unnecessary burdens on both participants and investigators, a pain point that has been increasingly perceived across the industry.
-
Principle 10: Focuses on Roles and Responsibilities, underscoring the necessity for clear and appropriately documented roles and responsibilities in clinical trials. This new principal introduces investigator delegation, which had not been clearly defined in R2, and emphasizes that sponsors and investigators will still retain overall responsibility for any transferred or delegated tasks. Agreements detailing these roles must be clearly defined and sponsors and investigators must maintain appropriate oversight of any and all delegated tasks. It is also worth noting the shift from the term “supervision” to “oversight” when referring to investigator responsibility, indicating increased accountability.
For the remaining 9 principles, the following updates are noted:
-
Principle 1: Ethical Principles – This principle combines several previous principles (2.1, 2.2, 2.3, 2.7, and 2.11), emphasizing periodic safety information reviews and the inclusion of representative participation.
-
Principle 2: Informed Consent – Information provided during the consent process must be clear and concise, including relevant details for participants. The use of technology, such as electronic ICFs, is permitted to reduce participant burden.
-
Principle 3: IRB/IEC Review – This principle mandates that periodic reviews follow applicable regulatory requirements, removing the previous requirement for annual reviews.
-
Principle 4: Science – Trials must be based on robust and current scientific knowledge, with periodic reviews to determine if modifications are needed based on new or changed information that may become available during a study. The goal is to ensure that the knowledge and experience surrounding the investigational product is relevant and sound.
-
Principle 5: Qualified Individuals – This principle expands on the concept that clinical trials should be conducted by qualified individuals, recognizing the need for different expertise across all phases of a trial.
-
Principle 6: Quality – The quality of trials should be fit for their purpose, with critical-to-quality (CtQ) factors identified prospectively and quality by design (QbD) focused on these factors.
-
Principle 8: Protocol – Protocols must be well-designed to ensure the protection of participants and the generation of reliable data. Protocols and ancillary documents must be clear, concise, and operationally feasible.
-
Principle 9: Reliable Results – The quality and volume of information generated in a trial should be fit for purpose to support reliable results and good decision-making. The use of computerized systems to support the garnering of results should also be fit for purpose and administered in a risk-proportionate manner.
-
This updated principle also notes the increased expectation for transparency in clinical trials, including timely registration on publicly accessible databases, public posting of trial results, and communication of results to participants.
-
-
Principle 11: Investigational Product (IP) – This principle outlines processes for IP management, expanding beyond acting in accordance with relevant Good Manufacturing Practice (GMP) standards, and now includes reference to proper handling, labeling and usage, while ensuring alignment with treatment assignments, including maintaining the blind where applicable, and ensuring overall IP quality for participants.
The visual below maps the changes in principles from R2 to R3.
Changes to industry-leading guidance such as ICH E6 can be overwhelming to navigate, which is why having a trusted partner with expertise in Good Clinical Practice (GCP) can be so valuable. MVG Consulting Services has been a valued partner throughout many industry changes and our GCP expertise allows us to provide continued guidance to our clients with these most recent changes. With this in mind, please reach out with any inquiries and our team will be happy to help.
MVG Consulting Services specializes in helping small to medium sized biotechnology and pharmaceutical companies with their clinical development programs and we can provide start-up and virtual companies with the necessary resources to begin their clinical development program.
Services include:
- Clinical Research Consulting Services
- Clinical Trial Development
- Project Rescue
- Inspection Readiness
MVG provides highly qualified clinical research professionals who seamlessly become a part of your project team. Additionally, we provide virtual companies with an entire project team that encompasses all aspects of clinical development.
We invite you to fill out the form below to learn more about how MVG Consulting can help you with your clinical devleopment needs.