Following the 21st Century Cures Act, passed in 2016, which aims at accelerating the development of medical products to allow innovation to reach patients more efficiently, the use of Real World Evidence (RWE) and Real World Data (RWD) has become increasingly impactful.
This increased importance stems from the potential use of RWE by regulatory bodies to; (a) support approvals for new indications and previously approved drugs and, (b) satisfy post-approval study requirements.
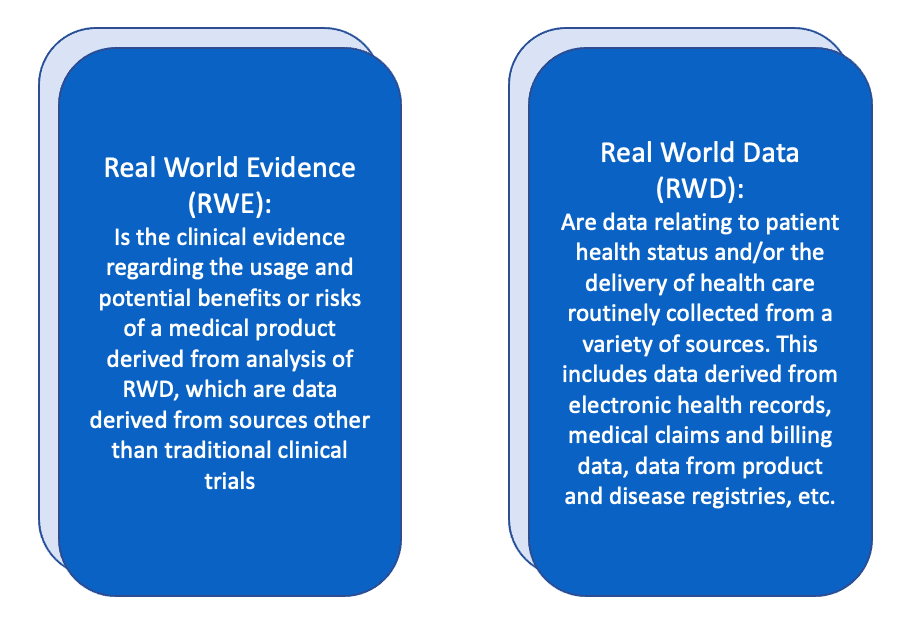
According to the FDA, Real World Evidence (RWE) and Real World Data (RWD) are defined as the following:
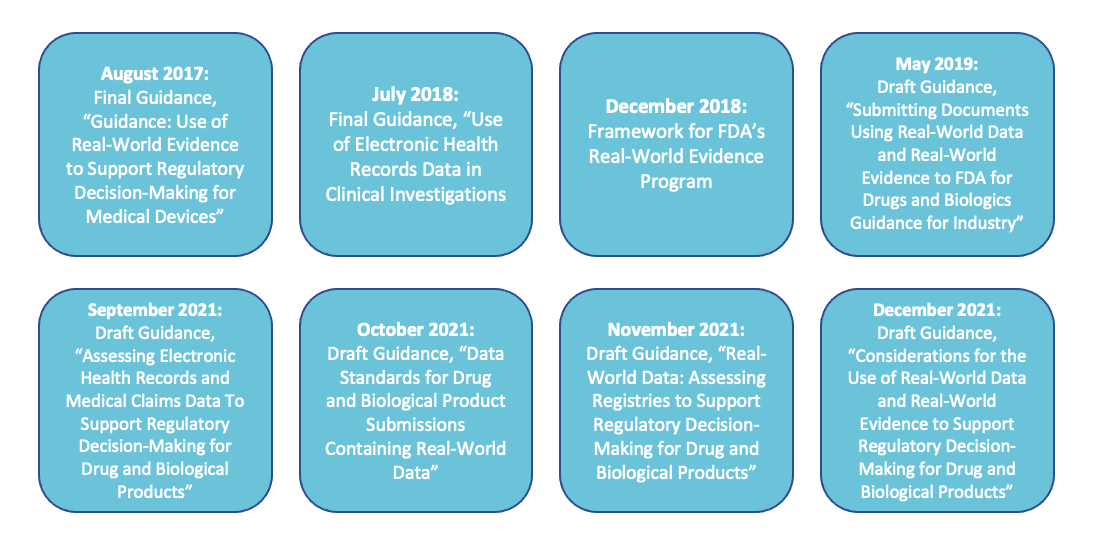
Since 2016, there have been 8 FDA Recommendations on RWE/RWD:
Alongside these FDA recommendations and the increased use of RWE/RWD in FDA submissions, considerations for BIMO inspections and data audits evaluating that data have become more important and central to the submission process. In cases where companies are outsourcing data management and other functionality, assurance of proper vendor oversight in these areas is even more important.
If you have concerns about your RWE/RWD vendor oversight or overall trial data integrity, MVG is the perfect partner to help work with you to find the right solution.
Contact MVG Consulting
MVG Consulting Services specializes in helping small to medium sized biotechnology and pharmaceutical companies with their clinical development programs and we can provide start-up and virtual companies with the necessary resources to begin their clinical development program.
Services include:
- Clinical Research Consulting Services
- Clinical Trial Development
- Project Rescue
- Inspection Readiness
MVG provides highly qualified clinical research professionals who seamlessly become a part of your project team. Additionally, we provide virtual companies with an entire project team that encompasses all aspects of clinical development.
We invite you to fill out the form below to learn more about how MVG Consulting can help you with your clinical devleopment needs.


