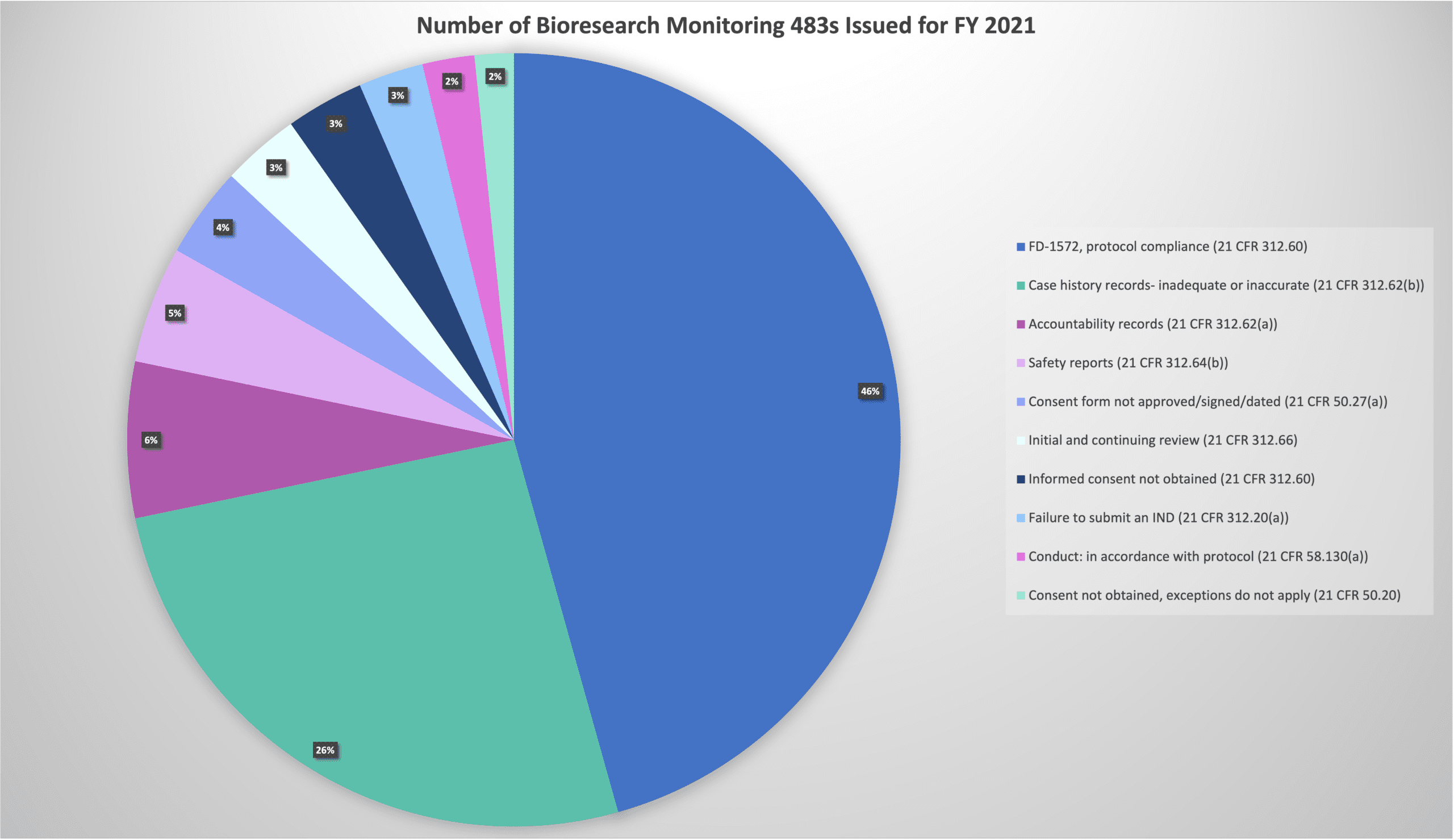
Every year, the FDA publishes records of the 483s issued during inspections, which provides a snapshot of the most prevalent inspection observations across the industry. Bioresearch Monitoring (BIMO) enforcement statistics relate directly to sites, sponsors, and vendors within clinical trials. As shown in the pie chart below, over 70% of all findings relate to protocol compliance and inadequate or inaccurate source documents.
Inspection Readiness doesn’t only apply to sponsors; clinical sites should also be inspection ready especially for pivotal studies that may be used in a marketing application. Sponsors should regularly review monitoring visit reports and conduct co-monitoring visits to ensure that the site is being adequately monitored and that any issues that arise are addressed in a timely manner. If sites have been identified for inspection by a regulatory agency, the site should undergo inspection readiness training.
Contact our team at MVG to discuss how we can help keep your studies on track by providing co-monitoring services, inspection readiness training and mitigation services for sites that are experiencing compliance issues.
MVG Consulting Services specializes in helping small to medium sized biotechnology and pharmaceutical companies with their clinical development programs and we can provide start-up and virtual companies with the necessary resources to begin their clinical development program.
Services include:
- Clinical Research Consulting Services
- Clinical Trial Development
- Project Rescue
- Inspection Readiness
MVG provides highly qualified clinical research professionals who seamlessly become a part of your project team. Additionally, we provide virtual companies with an entire project team that encompasses all aspects of clinical development.
We invite you to fill out the form below to learn more about how MVG Consulting can help you with your clinical devleopment needs.

