Risk Evaluation and Mitigation Strategies (REMS) programs are required by the US FDA to monitor certain medications that have high potential for serious adverse events. The FDA determines if a REMS is necessary during the approval process for a new product and if deemed necessary, the drug company is responsible for developing and maintaining the REMS program.
As of 1 April, 2025 there have been five new REMS programs approved thus far this year: Conexxence, Miudella, Ospomyv, PS-Pomalidomide and Stoboclo. Compared to the seven programs approved in 2024, it is likely that the number of new REMS programs to come in the remainder of 2025 will surpass that total.
When a newly approved product requires a REMS program, the specifics of the program can be presented to health care providers with either a Communication Plan and/or Elements to Assure Safe Use (ETASU). The latter provides more specific actions or interventions required to ensure safe prescribing and dispensing of the product and is considered to have a greater stringency of oversight.
Of the five newly approved programs in 2025, only two have ETASU requirements with the remaining three requiring only Communication Plans. As for the year prior, six of the seven programs approved had ETASU while only one required a Communication Plan. In 2023, 11 new programs were approved and all required ETASU, with one also requiring a Communication Plan. Similar trends were seen in 2022 and 2021 as well, with all approved REMS having ETASU requirements. The graphic below highlights the changes in the percent of approved REMS with ETASU vs. Communication Plans in the last five years. Note that some programs were approved with both ETASU and a Communication Plan.
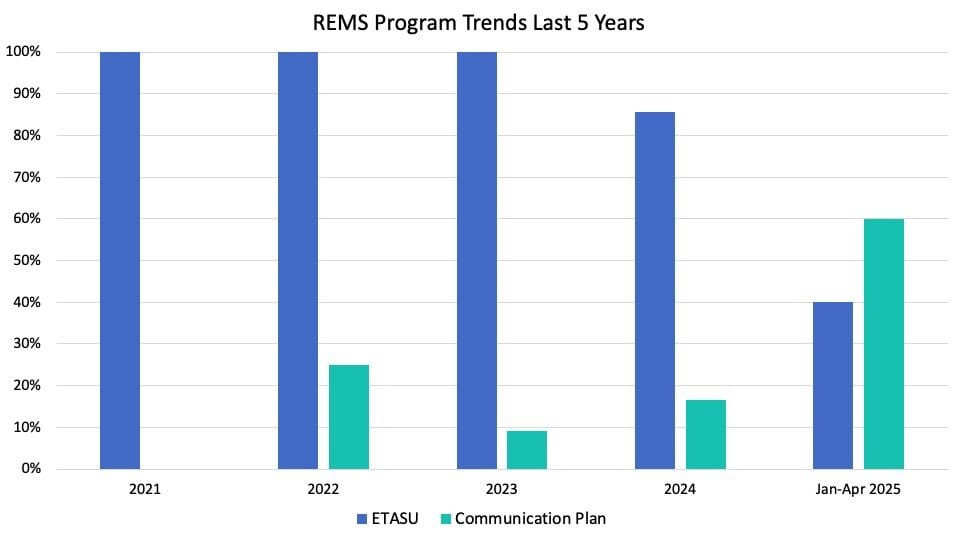
This shift in the current year of most newly approved REMS programs requiring only Communication Plans, could imply a shift in how new programs are being approved. For newly approved products with REMS required, the programs with only Communication Plans imply less strict oversight requirements. This could be occurring purely by chance, with the products themselves being less risky but still requiring a REMS. Alternately, the FDA could be getting stricter in their oversight requirements, so that a product that may have previously not required a REMS at all, now does require one but only with a Communication Plan.
The potential shift in REMS requirements bears watching to see if the trend continues. MVG Consulting, a proven provider of REMS program services, keeps close watch of the changes in these important programs and overall trends in the REMS space. If you already have an active REMS program in place or have an upcoming product approval with REMS requirements, MVG can be a great resource in helping to navigate how to both set up and manage your program.
MVG Consulting Services specializes in helping small to medium sized biotechnology and pharmaceutical companies with their clinical development programs and we can provide start-up and virtual companies with the necessary resources to begin their clinical development program.
Services include:
- Clinical Research Consulting Services
- Clinical Trial Development
- Project Rescue
- Inspection Readiness
MVG provides highly qualified clinical research professionals who seamlessly become a part of your project team. Additionally, we provide virtual companies with an entire project team that encompasses all aspects of clinical development.
We invite you to fill out the form below to learn more about how MVG Consulting can help you with your clinical devleopment needs.


